
Deciphering Science's Secrets: Lifespan, UPR, and Lysosomal Pathwayswith Dr. Arwen Gao on The VitaDAO Aging Science Podcast


In today’s podcast I talked to Dr. Arwen Gao about the working conditions in science and, of course, also about her research. First, we talked about scholarships, strikes, unions and bureaucracy before moving on to our research related topics like antibiotics that extend lifespan, the unfolded protein response (UPR) and a novel lysosomal surveillance pathway called LySR that she helped to identify.
You may want to skip to the first 20 minutes if you only want to hear about the hard science rather than our discussion of working conditions and the day-to-day life of a scientist.
Short Bio – Dr. Arwen Gao
Arwen was awarded an AMC PhD scholarship to pursue her PhD thesis, focusing on the metabolic control of aging in C. elegans, and earned her PhD degree in the fall of 2018 at the University of Amsterdam in the Netherlands. She continued her research on mitochondrial stress response and its role in metabolism and aging, under the supervision of Prof. Johan Auwerx (EPFL, Switzerland) and supported by a post-doctoral fellowship (2019 Accelerator price winner). After this postdoc, she moved back to the Netherlands, where she is currently a junior group leader at the Amsterdam UMC - Location AMC. Her research focuses on lysosomes, lipids, aging, and translational projects that can benefit patients with lysosome disorders.
A few helpful notes follow below explaining the concepts behind the topics we covered.
Worms as a model in aging research
There are many good reasons to use the worm (C. elegans) for preclinical aging research. For one, it is cheap to study and requires no ethics approval. These worms have a conveniently short lifespan of around 30 days and well understood genomes. In addition, many tools are available to manipulate their genomes including RNA-interference libraries to study the knock-down of genes in a high-throughput fashion.
There is also a surprising amount of conservation between human and worm genomes. Our genes often are very similar to worm genes suggesting that worms might be a suitable model to study some aspects of human aging. For example, FoxO signaling and the Igf-1 pathway have been first identified in worms and later confirmed to be important longevity pathways in mammals as well.
In the podcast we discussed how worm researchers also struggle with the same problems as mouse researchers. Similarly, to the situation with mice, most worm aging research is done in a single, highly lab-adapted and inbred strains. The one we often use is called N2 (first isolated in “Bristol”). We discussed the problems with inbred mice in a recent podcast with Rich Miller and in this podcast I discuss the C. elegans perspective with Arwen Gao and some solutions to this, including the generation of inbred, yet diverse, recombinant “panels”, composed of different strains with varying genetics. A simpler - somewhat less sophisticated - solution is to use at least two different strains to confirm your findings.
UPR and doxycycline - an unfolding story
Folding is the process by which linear protein chains are assembled into functioning 3D structures. Whenever something goes wrong with this folding process, this activates a so-called unfolded protein response (UPR). There are at least two distinct unfolded protein responses that occur in different cellular compartments, one in the mitochondria termed (UPRmt) and the other the canonical UPR in the endoplasmic reticulum (ER). Conceptually, the UPR can be considered a “cry for help” from an organelle. Since eukaryotic cells are very large and compartmentalized there is a need for this kind of cross-talk between different organelles and the nucleus which orchestrates said help (via mRNA transcription).
To make matters more confusing, the UPR, can be considered as part of the integrated stress response (ISR) which downstream is characterized by phosphorylation of a translational initiation factor (eIF2alpha) leading to selective translation of certain proteins, including the famous ATF4 transcription factor which is elevated in long-lived mouse models. Another way of looking at this is in terms of partial overlap. One branch of the canonical UPR, acting via a protein called PERK, is also an arm of the integrated stress response. However, the UPR has many other outputs and the integrated stress response has many other inputs, including the UPRmt.
Intriguingly, doxycycline is an antibiotic that activates the UPRmt thereby extending lifespan in C. elegans as shown by Gao et al. (2022). This is consistent with a naïve notion that improved folding and the UPR stress response should benefit lifespan. Since this is real biology, however, it is also the place where intuitions go to die. Other work suggests that inhibition of the integrated stress response is beneficial, at least in worms (Derisbourg 2021). This is the article I mentioned during the podcast from the Denzel group. While interesting, this does not – and should not – dissuade us from putting this idea to the test in the mammalian system. And, indeed, the famous mouse interventions testing program (ITP) is running a cohort with an integrated stress response activator (galofuginone) in mice to see whether it will extend lifespan.
We live in interesting times indeed!
For further reading we have attached links to some freely accessible reviews.
Melber and Haynes 2018 and Pakos‐Zebrucka et al. 2016 (here esp. Fig. 1) under further reading.
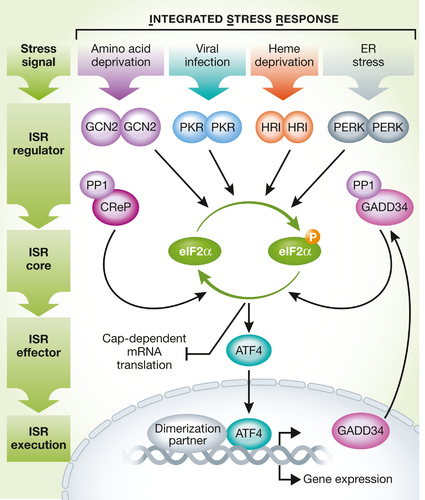
You can consider the UPR as one input for the integrated stress response. Source: Pakos‐Zebrucka et al. 2016, https://www.embopress.org/doi/full/10.15252/embr.201642195
The lysoosomal surveillance response – just another cry for help?
Arwen and others discovered a new pathway responsible for sensing lysosomal stress when they knocked down different VATPase subunits in the worm (Li et al. 2022). This pathway appears to be independent of autophagy, mTOR and the TFEB biogenesis pathway (called HLH-30 in the worm). While it is not entirely clear how the signal is transmitted from the lysosome to nucleus, they do show that it converges on a specific transcription factor from the GATA family. One can speculate that VATPase knock-down leads to reduced acidification and proteolysis in the lysosome which somehow leads to nuclear GATA translocation. While superficially, this may not be the whole story since only specific VATPase subunits seem to be involved in triggering the LysR. Hopefully, once we figure out the players we will be able to use this knowledge to generate novel autophagy inducers to promote lifespan and health.
Scientific research - between madness and dedication
In this podcast we also talk about the working conditions of scientists and PhD students. Arwen mentioned that the conditions are quite favorable in the Netherlands and in the labs where she worked. However, we are also aware of horror stories like postdocs who were regularly "sleeping in the lab" (undisclosed German lab) or students "buying groceries" for their supervisor (somewhere in the Netherlands or Switzerland, presumably). While we do both agree that passion is an important ingredient to a science career, (self-)exploitation should not be part of the deal. Sadly, these stories are more common than they should be. Grad school in the USA, for example, has the reputation of being very taxing. Here as told from the perspective of chemists:
https://www.science.org/content/blog-post/more-grad-school-pressures
https://www.science.org/content/blog-post/industry-vs-academia-mental-aspect
http://notthelab.blogspot.com/2013/01/is-graduate-school-in-chemistry-bad-for.html
Personally, I believe society should value scientists more than we currently do and pay significantly higher salaries. Whenever the pay is high, there is less exploitation of cheap labor and more automation of tedious labor.
However, not everything is bleak and we do not want to discourage anyone from pursuing a career in science. Conditions are improving and they are already quite good at certain institutions and in some countries. If you want to learn more which countries have a relaxed work culture and good pay, this podcast might be for you!
The host – brief bio

Kamil Pabis, MSc is an aging researcher and longevity advocate with several years of experience in the aging field that spans multiple countries. Among other projects, Kamil worked on long-lived dwarf mice in Austria, on mitochondrial disease and aging in the UK, and finally on the bioinformatics of aging in Germany and Singapore. Presently, he is involved in several projects related to science communication and translational aging research.
References and further reading
Derisbourg, Maxime J., et al. "Mutagenesis screen uncovers lifespan extension through integrated stress response inhibition without reduced mRNA translation." Nature Communications 12.1 (2021): 1678.
Arwen Gao at ARDD2022: A lysosomal surveillance response (LySR) that extends healthspan
https://www.youtube.com/watch?v=TiIs2stK2Iw&t=612s
see also biorxiv: https://www.biorxiv.org/content/10.1101/2022.06.13.495962v1
Gao, Arwen W., et al. "Multi-omics analysis identifies essential regulators of mitochondrial stress response in two wild-type C. elegans strains." Iscience 25.2 (2022): 103734.
Melber, Andrew, and Cole M. Haynes. "UPRmt regulation and output: a stress response mediated by mitochondrial-nuclear communication." Cell research 28.3 (2018): 281-295.
https://www.nature.com/articles/cr201816
Pakos‐Zebrucka, Karolina, et al. "The integrated stress response." EMBO reports 17.10 (2016): 1374-1395.
https://www.embopress.org/doi/full/10.15252/embr.201642195


